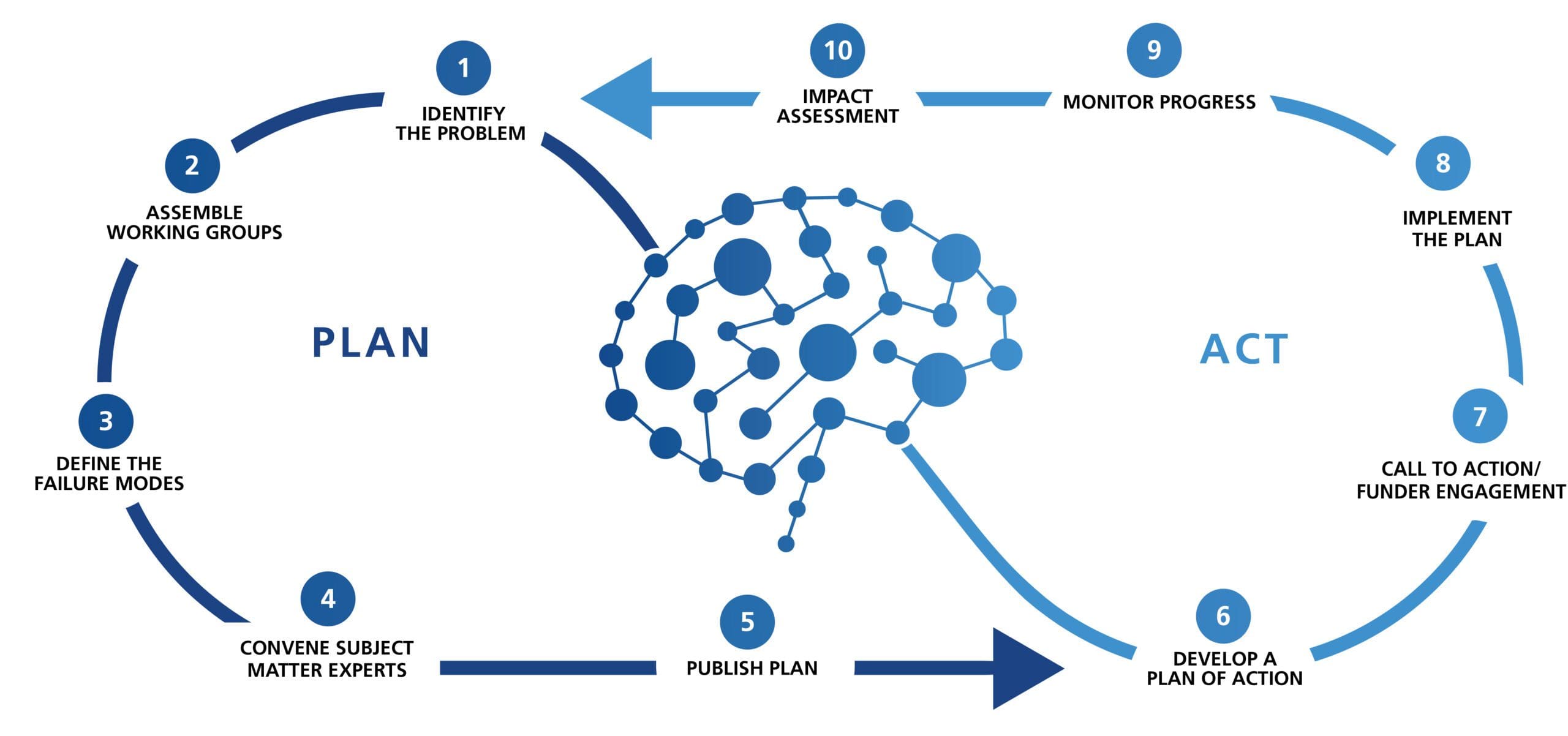
Individual Membership (Free)
Membership to the TBI Action Alliance for individuals is free of charge.
Your membership benefits include:
- Newsletter – updates on the latest progress & findings in TBI
- Surveys – opportunities to provide your input and feedback on a broad range of TBI topics
- Your Voice – weigh in on The Precision Research Roadmap priorities – your voice matters!
- Clinical Trial Information – learn about the latest studies and opportunities to participate in research
- Webinars – member-only invitations to learn more about TBI from the experts
To support the efforts of the TBI Action Alliance, and to help us drive a new era of research for TBI and advance a first generation of FDA-approved diagnostics and treatments, we ask that you consider making a tax-deductible donation in the amount of your choosing.
Please use the link below to make a one-time or recurring donation to support the TBI Action Alliance.
Donate Now
Trainee (students, post-doctoral) Membership ($50/year)
Your annual professional membership donation will help us drive a new era of research for TBI and advance a first generation of FDA-approved diagnostics and treatments.
Your membership benefits include:
- Newsletter – updates on the latest progress & findings in TBI
- Surveys – opportunities to provide your expertise on a broad range of TBI topics
- Grant Opportunities – learn about new research program and funding opportunities
- Webinars – member-only invitations to join online workshops and webinars
- Portal – access to the TBI Action Alliance Portal
Academic/Professional Membership ($500/year)
Your annual professional membership donation will help us drive a new era of research for TBI and advance a first generation of FDA-approved diagnostics and treatments.
Your membership benefits include:
- Newsletter – updates on the latest progress & findings in TBI
- Surveys – opportunities to provide your expertise on a broad range of TBI topics
- Grant Opportunities – learn about new research program and funding opportunities
- Webinars – member-only invitations to join online workshops and webinars
- Portal – access to the TBI Action Alliance Portal