70 Million
Individuals worldwide suffer a traumatic brain injury (TBI) each year.
>310,100
Military service members were affected by TBIs between 2000-2015 – it’s the most common traumatic injury in the U.S. military.
>80,000
The number of emergency department visits that happen each year from TBIs in adults over 65 due to falls.
Americans pour ourselves into everything we do.
We’re dreamers. Builders. And innovators.
Whether it’s winning a weekend game of football, driving 3000-miles on a cross country road trip to see the glory of our national parks, enjoying an afternoon on the monkey bars with friends, or putting in a hot day to build our roads and cities or protect our citizens, we live life with joy, passion, heart… and a little bit of daring.

Become a member of the alliance
~475,000
Children (0-14 years old) suffer a TBI each year due to falls and sports related accidents.
5.3 Million
Americans are living with a long-term disability due to a TBI.
$50 Billion
In 2015, the cost for direct TBI medical care in the U.S. was estimated at more than $50 Billion per year.

But when we live life to its fullest, accidents can and do happen.
In fact, some 5.3 million of us every year experience head trauma from falls, car accidents, collisions, flying construction debris, or military engagements. And head impact can cause injury to the brain, which in turn can cause depression, anxiety, suicide, PTSD and other conditions. Today, more than 5.3 million of us are living with TBIs.
Help accelerate solutions for brain injury
~$203 Million
The annual investment by the NIH in TBI-related research programs, representing less than half a percent of NIH’s $45 Billion budget.
1.5-2.8
Times more likely someone with a history of TBI or PTSD will attempt suicide compared to those without the diagnosis.
5.3 Million
The number of Americans with traumatic brain injuries that we can help each year with your contributions.
We’re connecting leading experts and catalyzing collaborative action.
The TBI Action Alliance will deliver improved brain health and outcomes for individuals who have experienced traumatic brain injuries through collective expertise and multi-sector action to deliver innovative solutions.
As part of the National Precision Research Roadmap for TBI, we’re working together to speed the development of diagnostics and treatments that improve the lives of the more than 5.3 million people living with TBI-related symptoms – including millions of veterans, first responders, athletes, elderly and children – and to help protect all Americans at risk for TBI from falls, accidents or sports-related impacts.

Join the alliance and become a partner
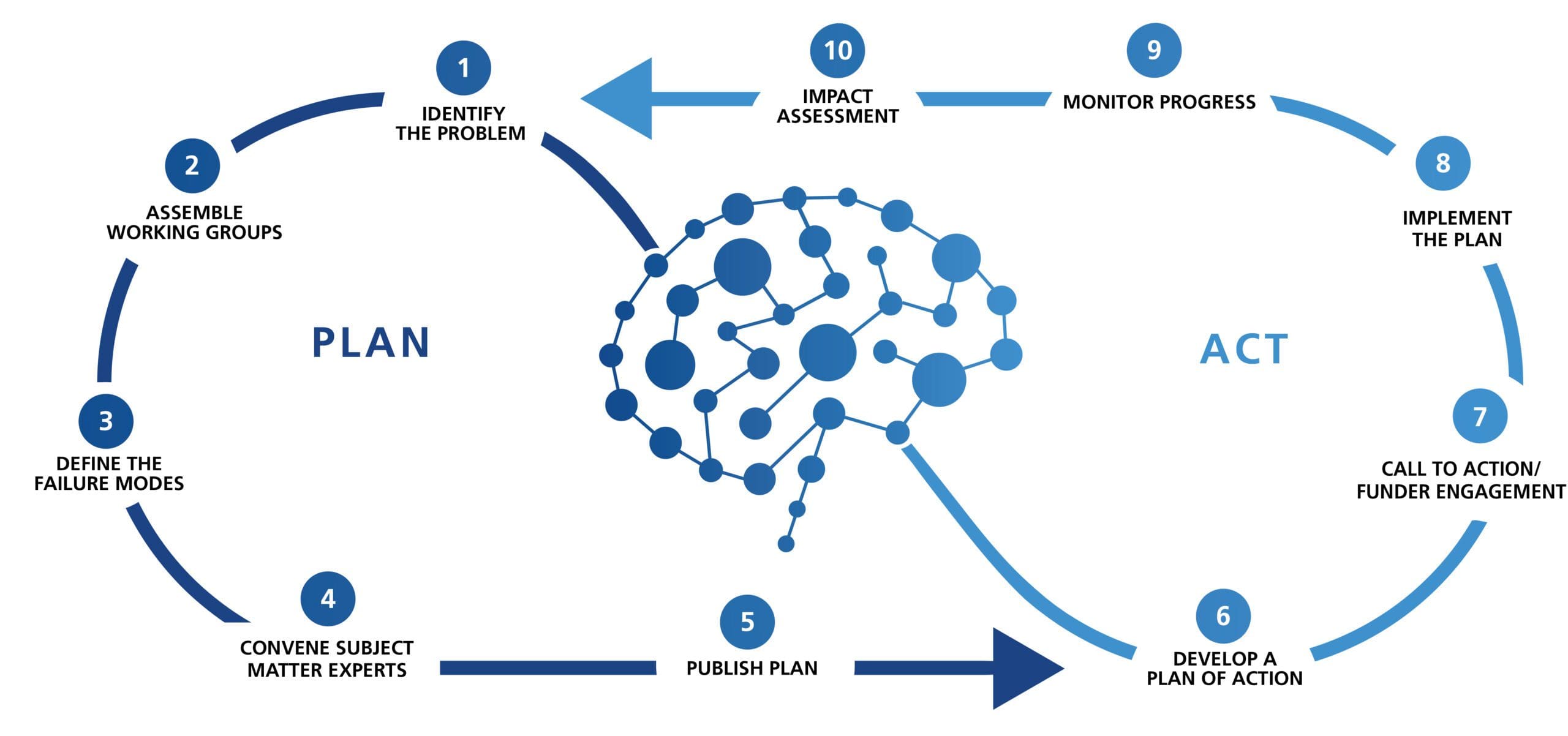
The Roadmap
Cohen Veterans Bioscience (CVB) established the Brain Trauma Blueprint – a framework for generating and executing precision research roadmaps.
Through this framework, CVB has convened TBI Researchers and other stakeholders at State of the Science Summits to identify and publish consensus challenges and recommendations for TBI research.